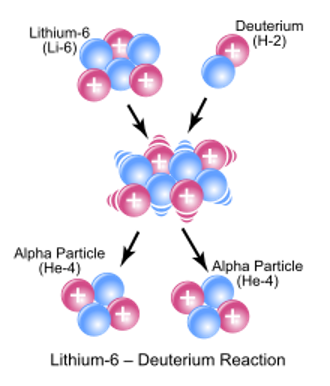Fusion on Earth vs Fusion in the Sun – How Do They Differ?
Comparing nature’s blueprint to human innovation.
The Big Fusion Machine in the Sky
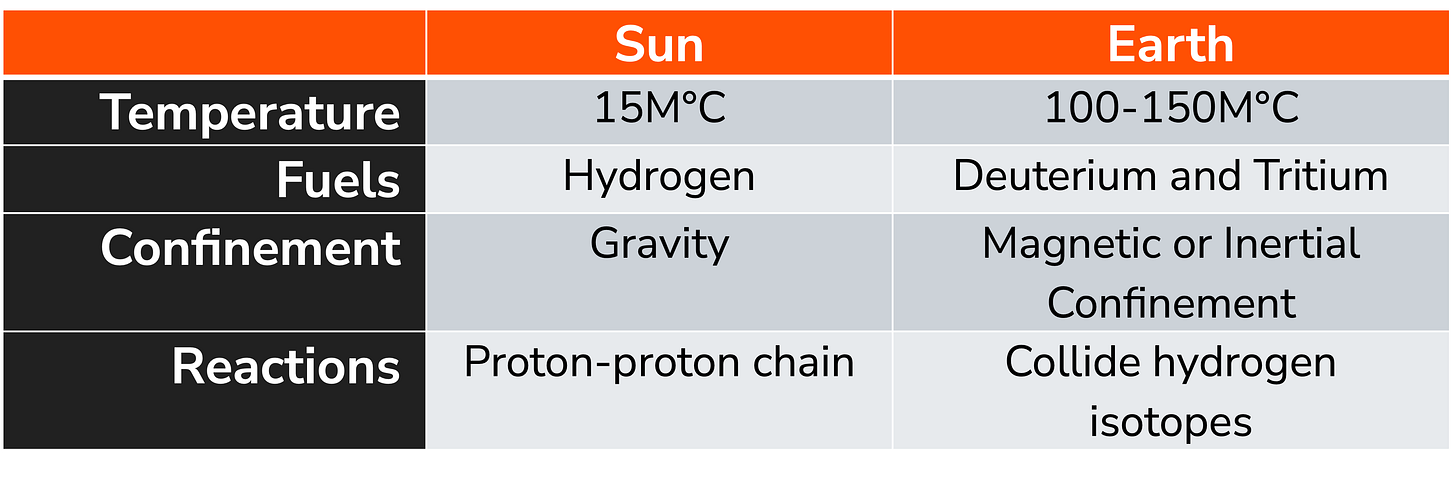
Last week, one of our articles discussed the differences between how national labs approach fusion and commercial entities approach fusion. In this article, let’s expand the scope and look at how our solar system’s most extensive fusion “machine” approaches fusion – the Sun. The Sun is an “average-ish” G-type main-sequence star (stars with a solar mass between 0/9 and 1.1 solar masses). Some key facts about our sun:
The Sun has a total mass of 1.9885 x 1030 kilograms (kg), with an equatorial radius of 6.957 x 108 meters (m) and an average density of 1.408 grams/cubic centimeter (g/cc3).
The Sun converts roughly 600 billion kilograms of hydrogen into helium every second, producing a core temperature of 15,700,000 degrees Kelvin (K) with a surface temperature of 5,800 K.
The Sun is roughly 4.6 billion years old. When most hydrogen fuses into helium, it will evolve into a red giant star between 4 and 7 billion years from now.
Solar Fusion Vs. Terrestrial Fusion
As we observed last week when comparing large-scale experimental fusion machines in national labs to private-company fusion efforts, it is essential to understand the fundamental differences between these solutions. The same is true regarding how the Sun creates fusion energy. One of the most significant differences between fusion in the Sun and fusion on Earth is the actual mechanism.
On Earth (including fusion in a thermonuclear weapon), most of the fusion occurs through the combination of deuterium (a hydrogen nucleus with one neutron) and tritium (a hydrogen nucleus with two neutrons), which is also known as D-T fusion. D-T fusion directly produces helium and one (1) high-energy neutron in a single step. In modern thermonuclear weapons, the lithium in the weapon’s “secondary” (lithium-6 deuteride) fuel produces the tritium for the D-T reaction.
Nuclear fusion, which powers the Sun and other stars, occurs differently in the Sun than in attempts to achieve fusion on Earth. The key differences lie in the conditions required for fusion and the methods used to achieve these conditions.
Fusion on the Sun occurs through a process called proton-proton fusion. In the Sun's core, where temperatures reach about 15 million degrees Celsius, hydrogen nuclei (protons) collide and fuse to form helium nuclei. This process is driven by the Sun's immense gravitational force, which creates extreme pressure and density, allowing the protons to overcome their mutual electrical repulsion. The fusion reactions release enormous amounts of energy in the form of heat and light, which radiates outward from the core and ultimately reaches Earth as sunlight.
Scientists aim to replicate fusion on Earth using a different approach due to the absence of intense gravitational forces. The most promising method involves heating a mixture of deuterium and tritium (hydrogen isotopes) to temperatures exceeding 150 million degrees Celsius, about ten times hotter than the Sun's core. At these extreme temperatures, the fuel becomes a hot, charged plasma. Powerful magnetic fields are used in tokamaks to contain this superheated plasma, which creates a "magnetic cage" to confine the particles. The goal is to maintain these conditions long enough for sufficient fusion reactions, producing more energy than is required to heat and confine the plasma.
The basic principle of fusion is the same. However, the conditions and methods required to achieve fusion on Earth differ substantially from those in the Sun, primarily because our planet lacks intense gravitational forces.
The Sun’s Approach to Fusion
Unlike on Earth, events that create deuterons (the nucleus of deuterium) in stars are rare, and the creation of tritons (the nucleus of tritium) is super-rare. This is why the Sun uses a process known as the “proton-proton” chain to produce fuse hydrogen into helium. Proton-proton fusion is the dominant form of fusion in stars with less than 1.3 solar masses; stars with greater than 1.3 solar masses predominantly utilize the carbon-nitrogen-oxygen (CNO) cycle, which uses these elements as a catalyst. In the Sun, roughly 99% of the energy output comes from the proton-proton chain, while the rest comes from the CNO cycle.
A proton-proton chain takes six (6) hydrogen nuclei and converts them into helium-4, two (2) hydrogen nuclei, and an assortment of gamma rays, positrons, and neutrinos. The steps in the proton-proton chain are as follows (see diagram to the right):
Two hydrogen nuclei, each consisting of a single proton, fuse into a deuteron. One of the two fused protons is converted into a neutron through beta plus decay, which produces the deuteron, a positron, an electron neutrino, and 1.442 MeV. This prolonged reaction (thousands of years or more) limits the rate at which the Sun burns its hydrogen.
The deuteron then fuses with another free proton to produce helium-3, producing a gamma ray and 5.493 MeV of energy. This happens in about one second within the core of the Sun.
In most cases, two helium-3 nuclei fuse into a helium-4 nucleus, shedding two protons and producing about 12.859 MeV of energy. This approach (known as the “p-p I chain”) occurs for about 83.3% of helium-3 nuclei, which have a half-life in the Sun’s core of about 400 years.
In the remaining 16.7% of cases that do not involve the p-p I chain, reactions involve the creation of beryllium (four protons, three neutrons), which decays into lithium-7 (three protons, four neutrons) that eventually combines with a proton to create two helium-4 nuclei. These reactions occur on the interior of the Sun’s core, where the temperatures (and pressures) are the highest.
The fusion reaction consumes four (4) protons and two electrons, creating helium-4 nuclei, two electron neutrinos, and 26.73 MeV of energy. Since some of the energy is contained in the neutrinos (which minimally interact with “normal matter”), a percentage of the 26.73 MeV is essentially “lost.”
Fusion on Earth
Nuclear fusion on Earth aims to replicate the energy-producing process that powers the Sun and other stars. However, because stars do not have intense gravitational forces, the approach on Earth differs significantly.
The fusion process on Earth typically involves the following key elements:
Fuel Selection - The most common fusion reaction pursued on Earth uses two hydrogen isotopes: deuterium and tritium. Deuterium can be extracted from seawater, while tritium is generated from lithium.
Plasma Creation - The fusion fuel is heated to extremely high temperatures, around 150 million degrees Celsius, about 10 times hotter than the Sun's core. At this temperature, the fuel becomes a plasma—a hot, charged gas of ions and electrons.
Confinement - To contain this superheated plasma, scientists use powerful magnetic fields in a tokamak device. This doughnut-shaped chamber employs magnetic fields to confine and control the plasma.
Fusion Reaction - Under these extreme conditions, the deuterium and tritium nuclei overcome their mutual electrical repulsion and fuse, releasing energy.
Achieving sustainable fusion on Earth faces several challenges:
Maintaining the incredibly high temperatures required for fusion.
Effectively confining the plasma using magnetic fields.
Developing materials that can withstand the intense conditions in a fusion reactor.
Generating more energy from the fusion reaction than is required to heat and confine the plasma.
Scientists and engineers worldwide are working on overcoming these challenges, with projects like ITER (International Thermonuclear Experimental Reactor) aiming to demonstrate the feasibility of fusion as a large-scale energy source.
Bringing The Fusion Process Down to Earth
Why is it important to understand the difference between what occurs in the Sun and artificial fusion reactions on Earth? It helps us understand the conditions and likelihood of artificially reproducing alternatives to the D-T cycle for fusion energy. For instance, we know from studies of “natural” fusion in stars that, while the P-P cycle is effective in the Sun, it requires extremely high temperatures and pressures equal to those at the Sun’s core. It also is, in terms of our lifetimes, too “slow” a reaction to be helpful to produce artificial fusion energy in a timeframe to be beneficial to humanity (remember that helium-3 atoms have an average half-life of 400 years in the conditions of the Sun’s core, and the creation of deuterons takes orders of magnitude longer than that. However, that is not to say that fusion reactions other than D-T could be helpful in the artificial creation of fusion energy.
One of the most interesting alternatives to D-T fusion is aneutronic fusion reactions in which free neutrons are not produced. Aneutronic fusion is attractive for two reasons: a) it reduces damage caused by neutrons to both fusion machine structural materials and to people, and b) it is far simpler to convert the energy from charged particles (protons and electrons) into electricity than it is to do so with uncharged particles such as neutrons. Conversely, the conditions required for successful aneutronic fusion (temperature and plasma density) are far more extreme than those required for D-T fusion.
The most-studied alternative to D-T fusion is the fusion of deuterium with lithium-6, as shown here. Lithium-6 (Li6) is a naturally occurring isotope of lithium, constituting between 1.9% and 7.8% of all lithium. In aneutronic fusion, Li6 fuses with deuterium nuclei to produce beryllium-8, which has a half-life of 8.19 x 10-17 seconds, meaning it spontaneously decays into two alpha particles (helium-4 nuclei). These positively charged alpha particles can generate electricity in several ways that are far easier than turning neutron kinetic energy into heat.
Of the aneutronic options on the right (D-T shown for reference in green), the D-He3 reaction has the lowest ignition energy at 58 keV, slightly overtaking the D-T reaction's energy. However, He-3 is extremely rare on the Earth and would either have to be produced in a fission reactor or mined off-earth (like on the Moon). The p-Li6 reaction requires a slightly higher ignition temperature (below 5X that of the D-T reaction). It is well-understood due to its importance in breeding tritium for thermonuclear weapons.
Similarly, the p-B11 reaction is also well-understood due to the high availability of boron as a fuel. However, the p-B11 ignition temperature is 9X that of the D-T reaction, well above the temperatures that can be achieved in today’s tokamaks. In these cases, inertial confinement fusion (ICF) approaches combining lasers and proton beams would likely be the most promising. However, ‘side’ reactions in the p-B11 reaction can produce high-energy neutrons, negating some of the value of the p-B11 approach to fusion.
Putting the Sun in a Can on Earth
Scientists and engineers are working tirelessly to harness the power of the Sun here on Earth through nuclear fusion, a process that promises nearly limitless clean energy. Unlike the Sun, where fusion occurs naturally due to immense gravitational forces, researchers on Earth must create extreme conditions to achieve fusion in a controlled environment. The most promising approach involves heating a mixture of deuterium and tritium (hydrogen isotopes) to temperatures exceeding 150 million degrees Celsius - about ten times hotter than the Sun's core. This superheated fuel becomes plasma and is confined using powerful magnetic fields in donut-shaped devices called tokamaks.
Recent breakthroughs have brought us closer to realizing fusion as a viable energy source. In 2022, scientists at the National Ignition Facility achieved a significant milestone by producing more energy from a fusion reaction than was directly used to initiate it. Additionally, advancements in high-temperature superconducting magnets allow for more compact fusion devices. Despite these achievements, challenges remain, including developing materials that can withstand the extreme conditions inside a fusion reactor and solving complex engineering problems to extract usable energy efficiently. As research progresses, public and private sectors invest heavily in fusion technology, with some optimistic projections suggesting commercial fusion power could be a reality within a decade.